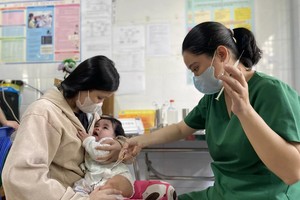
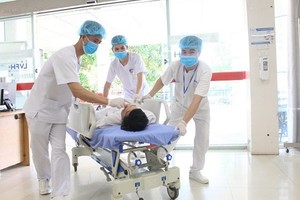
After a thorough evaluation of its standards for efficacy, safety and quality, Vero Cell vaccine has been approved for uses by IVAC.
The first batch of one million doses of Vero Cell vaccines is part of the five million doses that Saigon Pharmaceutical Company Limited (Sapharco) had negotiated with partner Sinopharm.
The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine (Vero Cell), produced by Beijing Bio-Institute of Biological Products Co Ltd, subsidiary of China National Biotec Group (CNBG).
On May 7, WHO listed the Sinopharm Covid-19 vaccine for emergency use, while Vietnam approved emergency use of the vaccine on June 4.